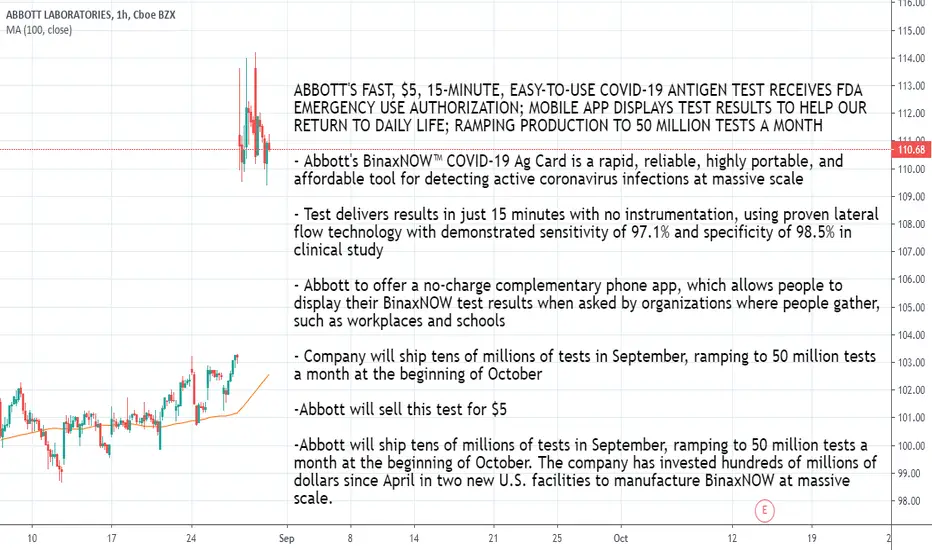
ABBOTT'S FAST, $5, 15-MINUTE, EASY-TO-USE COVID-19 ANTIGEN TEST RECEIVES FDA EMERGENCY USE AUTHORIZATION; MOBILE APP DISPLAYS TEST RESULTS TO HELP OUR RETURN TO DAILY LIFE; RAMPING PRODUCTION TO 50 MILLION TESTS A MONTH
- Abbott's BinaxNOW™ COVID-19 Ag Card is a rapid, reliable, highly portable, and affordable tool for detecting active coronavirus infections at massive scale
- Test delivers results in just 15 minutes with no instrumentation, using proven lateral flow technology with demonstrated sensitivity of 97.1% and specificity of 98.5% in clinical study
- Abbott to offer a no-charge complementary phone app, which allows people to display their BinaxNOW test results when asked by organizations where people gather, such as workplaces and schools
- Company will ship tens of millions of tests in September, ramping to 50 million tests a month at the beginning of October
-Abbott will sell this test for $5
-Abbott will ship tens of millions of tests in September, ramping to 50 million tests a month at the beginning of October. The company has invested hundreds of millions of dollars since April in two new U.S. facilities to manufacture BinaxNOW at massive scale.
abbott.mediaroom.com/2020-08-26-Abbotts-Fast-5-15-Minute-Easy-to-Use-COVID-19-Antigen-Test-Receives-FDA-Emergency-Use-Authorization-Mobile-App-Displays-Test-Results-to-Help-Our-Return-to-Daily-Life-Ramping-Production-to-50-Million-Tests-a-Month
This is not a test for asymptomatic people. I’ll perk up when the asymptomatic test launches.
twitter.com/Casey21288080/status/1298808389326090241
- Abbott's BinaxNOW™ COVID-19 Ag Card is a rapid, reliable, highly portable, and affordable tool for detecting active coronavirus infections at massive scale
- Test delivers results in just 15 minutes with no instrumentation, using proven lateral flow technology with demonstrated sensitivity of 97.1% and specificity of 98.5% in clinical study
- Abbott to offer a no-charge complementary phone app, which allows people to display their BinaxNOW test results when asked by organizations where people gather, such as workplaces and schools
- Company will ship tens of millions of tests in September, ramping to 50 million tests a month at the beginning of October
-Abbott will sell this test for $5
-Abbott will ship tens of millions of tests in September, ramping to 50 million tests a month at the beginning of October. The company has invested hundreds of millions of dollars since April in two new U.S. facilities to manufacture BinaxNOW at massive scale.
abbott.mediaroom.com/2020-08-26-Abbotts-Fast-5-15-Minute-Easy-to-Use-COVID-19-Antigen-Test-Receives-FDA-Emergency-Use-Authorization-Mobile-App-Displays-Test-Results-to-Help-Our-Return-to-Daily-Life-Ramping-Production-to-50-Million-Tests-a-Month
This is not a test for asymptomatic people. I’ll perk up when the asymptomatic test launches.
twitter.com/Casey21288080/status/1298808389326090241
Penafian
Maklumat dan penerbitan adalah tidak dimaksudkan untuk menjadi, dan tidak membentuk, nasihat untuk kewangan, pelaburan, perdagangan dan jenis-jenis lain atau cadangan yang dibekalkan atau disahkan oleh TradingView. Baca dengan lebih lanjut di Terma Penggunaan.
Penafian
Maklumat dan penerbitan adalah tidak dimaksudkan untuk menjadi, dan tidak membentuk, nasihat untuk kewangan, pelaburan, perdagangan dan jenis-jenis lain atau cadangan yang dibekalkan atau disahkan oleh TradingView. Baca dengan lebih lanjut di Terma Penggunaan.