Merck (NYSE:  MRK) has secured a major regulatory milestone after the European Commission approved a new subcutaneous version of Keytruda, the company’s flagship cancer therapy. This marks a significant expansion for one of the world’s best-selling immunotherapy drugs, previously only administered intravenously. The new formulation allows injections under the skin, giving patients faster, more convenient access to treatment while reducing strain on hospital infusion facilities.
MRK) has secured a major regulatory milestone after the European Commission approved a new subcutaneous version of Keytruda, the company’s flagship cancer therapy. This marks a significant expansion for one of the world’s best-selling immunotherapy drugs, previously only administered intravenously. The new formulation allows injections under the skin, giving patients faster, more convenient access to treatment while reducing strain on hospital infusion facilities.
The approval applies to all adult indications for which Keytruda is already authorized in the European Union. Keytruda works by activating the body’s immune system to identify and attack cancer cells, and it remains a cornerstone therapy across multiple cancer types. Merck reported more than $23.3 billion in Keytruda sales during the first nine months of the year, underscoring the drug’s central role in the company’s revenue base. The new delivery method may further strengthen market demand, especially in regions prioritizing efficiency and patient comfort.
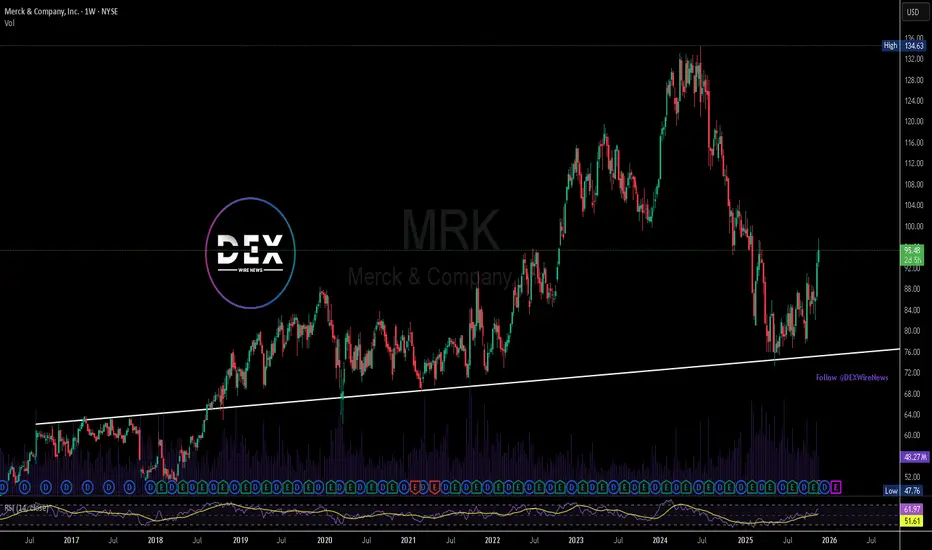
From a technical standpoint, Merck’s stock continues to trade in a bullish structure. Price action remains firmly above a long-standing ascending support trendline that has held since 2019. As long as this structure remains intact, the next major long-term upside target sits at the $134 level, a zone supported by higher-timeframe momentum. A temporary pullback is possible, and any retracement could provide a retest of the ascending support before buyers attempt another continuation.
Overall, Merck’s regulatory win adds fundamental strength to an already constructive technical outlook. With Keytruda’s expanded accessibility and consistent revenue performance, long-term sentiment appears supportive. Bulls will want price to stay above the structural trendline to maintain the pathway toward the $134 target.
The approval applies to all adult indications for which Keytruda is already authorized in the European Union. Keytruda works by activating the body’s immune system to identify and attack cancer cells, and it remains a cornerstone therapy across multiple cancer types. Merck reported more than $23.3 billion in Keytruda sales during the first nine months of the year, underscoring the drug’s central role in the company’s revenue base. The new delivery method may further strengthen market demand, especially in regions prioritizing efficiency and patient comfort.
From a technical standpoint, Merck’s stock continues to trade in a bullish structure. Price action remains firmly above a long-standing ascending support trendline that has held since 2019. As long as this structure remains intact, the next major long-term upside target sits at the $134 level, a zone supported by higher-timeframe momentum. A temporary pullback is possible, and any retracement could provide a retest of the ascending support before buyers attempt another continuation.
Overall, Merck’s regulatory win adds fundamental strength to an already constructive technical outlook. With Keytruda’s expanded accessibility and consistent revenue performance, long-term sentiment appears supportive. Bulls will want price to stay above the structural trendline to maintain the pathway toward the $134 target.
⭐⭐⭐ Sign Up for Free ⭐⭐⭐
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Chat Room >> discord.gg/x3EQven3ZY
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Chat Room >> discord.gg/x3EQven3ZY
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
Penerbitan berkaitan
Penafian
Maklumat dan penerbitan adalah tidak bertujuan, dan tidak membentuk, nasihat atau cadangan kewangan, pelaburan, dagangan atau jenis lain yang diberikan atau disahkan oleh TradingView. Baca lebih dalam Terma Penggunaan.
⭐⭐⭐ Sign Up for Free ⭐⭐⭐
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Chat Room >> discord.gg/x3EQven3ZY
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Chat Room >> discord.gg/x3EQven3ZY
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
Penerbitan berkaitan
Penafian
Maklumat dan penerbitan adalah tidak bertujuan, dan tidak membentuk, nasihat atau cadangan kewangan, pelaburan, dagangan atau jenis lain yang diberikan atau disahkan oleh TradingView. Baca lebih dalam Terma Penggunaan.