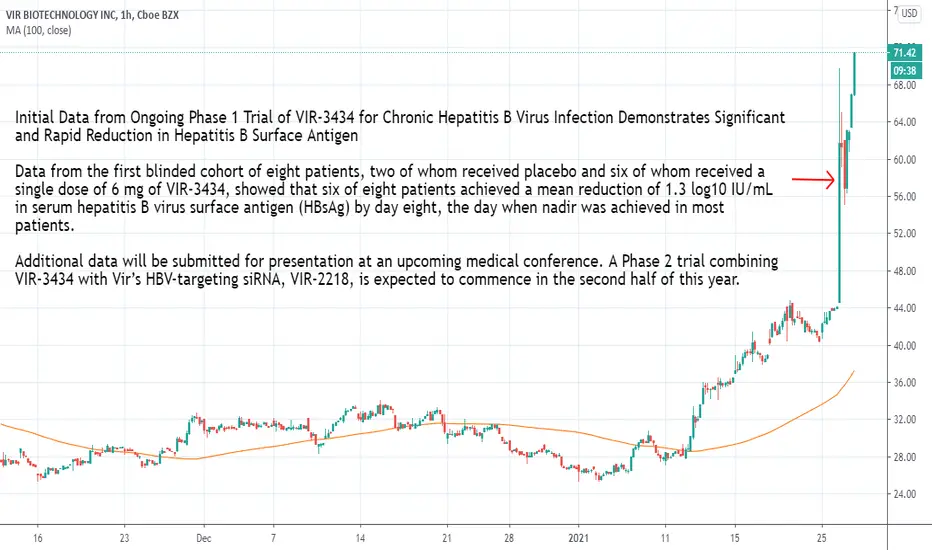
Initial Data from Ongoing Phase 1 Trial of VIR-3434 for Chronic Hepatitis B Virus Infection Demonstrates Significant and Rapid Reduction in Hepatitis B Surface Antigen
Data from the first blinded cohort of eight patients, two of whom received placebo and six of whom received a single dose of 6 mg of VIR-3434, showed that six of eight patients achieved a mean reduction of 1.3 log10 IU/mL in serum hepatitis B virus surface antigen (HBsAg) by day eight, the day when nadir was achieved in most patients.
Additional data will be submitted for presentation at an upcoming medical conference. A Phase 2 trial combining VIR-3434 with Vir’s HBV-targeting siRNA, VIR-2218, is expected to commence in the second half of this year.
finance.yahoo.com/news/initial-data-ongoing-phase-1-130000526.html
Data from the first blinded cohort of eight patients, two of whom received placebo and six of whom received a single dose of 6 mg of VIR-3434, showed that six of eight patients achieved a mean reduction of 1.3 log10 IU/mL in serum hepatitis B virus surface antigen (HBsAg) by day eight, the day when nadir was achieved in most patients.
Additional data will be submitted for presentation at an upcoming medical conference. A Phase 2 trial combining VIR-3434 with Vir’s HBV-targeting siRNA, VIR-2218, is expected to commence in the second half of this year.
finance.yahoo.com/news/initial-data-ongoing-phase-1-130000526.html
Penafian
Maklumat dan penerbitan adalah tidak bertujuan, dan tidak membentuk, nasihat atau cadangan kewangan, pelaburan, dagangan atau jenis lain yang diberikan atau disahkan oleh TradingView. Baca lebih dalam Terma Penggunaan.
Penafian
Maklumat dan penerbitan adalah tidak bertujuan, dan tidak membentuk, nasihat atau cadangan kewangan, pelaburan, dagangan atau jenis lain yang diberikan atau disahkan oleh TradingView. Baca lebih dalam Terma Penggunaan.